tima's DIY RCM
- Thread starter tima
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
David:Neil, thanks for the detailed response. I had tried a 3 fan computer radiator before but I found that the higher readings on the TDS meter indicated there could be some interaction between the cleaning solution and the radiator.
This is the radiator I used: https://tinyurl.com/89npepuc
It is copper so that may be the issue.
If I can just drop a radiator between the pump and filter and the pump will keep things flowing I am open to trying one of your solutions. Even with the 70% power the temps were too high to do multiple cleanings back to back.
The copper radiator will corrode releasing copper ions with DIW and you will see that as an increase in TDS. Aluminum looks to be compatible.
Please note - the radiator is installed after the filter. This is very important. If the radiator is installed between the pump and filter, as the filter loads and pump pressure increases the radiator will see that higher pressure, and these low cost aluminum radiators can be over-pressurized by the Shurflo pump.
Please tell how much the temp changes after 10min at 70%/37kHz and then after 10min at 100%/80Khz, and I will search out a radiator/fan system.
These radiators come in different configurations - some just have threaded female fittings so you pick the male bared fittings of your choice. Otherwise, there are always barb hose reducers -Quickun1/2" to 3/8" Barb Reducer Hose ID Reducing Barbed Splicer, Round Union Fitting Intersection/Split Water/Fuel/Air: Amazon.com: Industrial & Scientific.It would also be helpful to find a radiator that has 3/8” fittings so all the tubing is that same.
Take care,
Neil
David (@dminches),
Executive summary: The Koolance Radiator, 2x120mm 18-FPI Aluminum, Part No. HX-720 appears to be best option. Move over two of the three 120mm fans from your copper unit - the fan bolting is standardized. You may be able move the hose fittings from your current unit to the Koolance unit - they use similar dimensional female ports, if not then Koolance has nice fittings - Rotary Elbow Barb Fitting for ID 10mm (3/8in), G 1/4 BSPP (koolance.com) which are nickel over brass which is good.
The Koolance products all have cut-sheets, so there is something to review and they appear to be technically competent, so here is the radiator - radiator-2-fan-120mm-18-fpi-aluminum (koolance.com). The fans from your current unit are likely no better than the lowest performance fans. Recognizing that the air to water temp will be 95/75 (11C delta) at highest and 86/75 (6C delta) at lowest, you should get about 200W cooling average. If this aluminum heat exchanger works with the fluid (no significant rise in TDS), but you do not get enough cooling, Koolance sells much higher flow 12VDC fans (as much as 2A draw) and with the best fans (they are $27/ea) you should get about 400W cooling avg.
Without going into a long a tedious discussion, Thermaltake the manufacture of your current 3x120mm fan copper radiator has an aluminum option, but is coated with zinc - not good for DIW. Amazon has a bunch of 2x120mm & 3x120mm aluminum radiators, the same unit is made by 2-3 different vendors (China common item) and there are no details, no web address, and I just do not trust that.
I did come across this test of Computer heat exchangers Radiator Heat Dissipation Testing - Overclockers from 20-yrs ago, and from this the Koolance is close in what they report. This testing did highlight the problem with liquid pressure drop. If you used your 3x120mm copper radiator with your prior Little Giant flow you probably did not get much flow and therefore not much cooling. The Shurflo pump will not be bothered much by the radiator pressure drop (loss) and so flow-wise - no problem.
So, to recap, my best recommendation for cost/performance is this Koolance Radiator, 2x120mm 18-FPI Aluminum
Part No. HX-720. Note they do not offer a 3x120mm aluminum radiator. And, as I said, if its materially compatible with the DIW (it should be, but there is some risk) there is the option to add more powerful fans to improve cooling. Note that if you proceed, when you 1st assemble operate (with the Tergitol degassed) for a couple hours (to flush out the radiator and condition the aluminum surface. Also operate the heater (start low) which can then tell how much cooling the radiator can provide.
If the Koolance aluminum radiator is not compatible, then the only option is stainless steel and the cost is now $600 at best.
Good Luck,
Neil
Executive summary: The Koolance Radiator, 2x120mm 18-FPI Aluminum, Part No. HX-720 appears to be best option. Move over two of the three 120mm fans from your copper unit - the fan bolting is standardized. You may be able move the hose fittings from your current unit to the Koolance unit - they use similar dimensional female ports, if not then Koolance has nice fittings - Rotary Elbow Barb Fitting for ID 10mm (3/8in), G 1/4 BSPP (koolance.com) which are nickel over brass which is good.
The Koolance products all have cut-sheets, so there is something to review and they appear to be technically competent, so here is the radiator - radiator-2-fan-120mm-18-fpi-aluminum (koolance.com). The fans from your current unit are likely no better than the lowest performance fans. Recognizing that the air to water temp will be 95/75 (11C delta) at highest and 86/75 (6C delta) at lowest, you should get about 200W cooling average. If this aluminum heat exchanger works with the fluid (no significant rise in TDS), but you do not get enough cooling, Koolance sells much higher flow 12VDC fans (as much as 2A draw) and with the best fans (they are $27/ea) you should get about 400W cooling avg.
Without going into a long a tedious discussion, Thermaltake the manufacture of your current 3x120mm fan copper radiator has an aluminum option, but is coated with zinc - not good for DIW. Amazon has a bunch of 2x120mm & 3x120mm aluminum radiators, the same unit is made by 2-3 different vendors (China common item) and there are no details, no web address, and I just do not trust that.
I did come across this test of Computer heat exchangers Radiator Heat Dissipation Testing - Overclockers from 20-yrs ago, and from this the Koolance is close in what they report. This testing did highlight the problem with liquid pressure drop. If you used your 3x120mm copper radiator with your prior Little Giant flow you probably did not get much flow and therefore not much cooling. The Shurflo pump will not be bothered much by the radiator pressure drop (loss) and so flow-wise - no problem.
So, to recap, my best recommendation for cost/performance is this Koolance Radiator, 2x120mm 18-FPI Aluminum
Part No. HX-720. Note they do not offer a 3x120mm aluminum radiator. And, as I said, if its materially compatible with the DIW (it should be, but there is some risk) there is the option to add more powerful fans to improve cooling. Note that if you proceed, when you 1st assemble operate (with the Tergitol degassed) for a couple hours (to flush out the radiator and condition the aluminum surface. Also operate the heater (start low) which can then tell how much cooling the radiator can provide.
If the Koolance aluminum radiator is not compatible, then the only option is stainless steel and the cost is now $600 at best.
Good Luck,
Neil
Good Luck,
Neil
Neil, your assistance and knowledge are invaluable. I am going to order the Koolance and let you now how things go after it is all set up.
Thanks again.
Neil, your assistance and knowledge are invaluable. I am going to order the Koolance and let you now how things go after it is all set up.
This will be an interesting experiment.
I did notice that by the end of 20 minutes (10 @ 37 Hz @ 70% and 10 @ 80 Hz @ 100%) the temperature was 35 degrees C instead of 33 or 34.
Is that using the tank heater set at 30° C?
What I've been doing lately is not using the heater for wash and letting ultrasonic action raise the temperature gradually. Towards the end of the 10 minute 80kHz (following the 37kHz cycle) the P120H thermometer says 33°-34° C.
I presume heating the solution has value to the extent the surfactant is more effective at a higher temp than room temperature. Here is a question for Neil (apologies if your book has the answer): At what temperature is Tergitol 15-S-9 most effective and does its effectiveness rise as the solution moves toward that temperature?
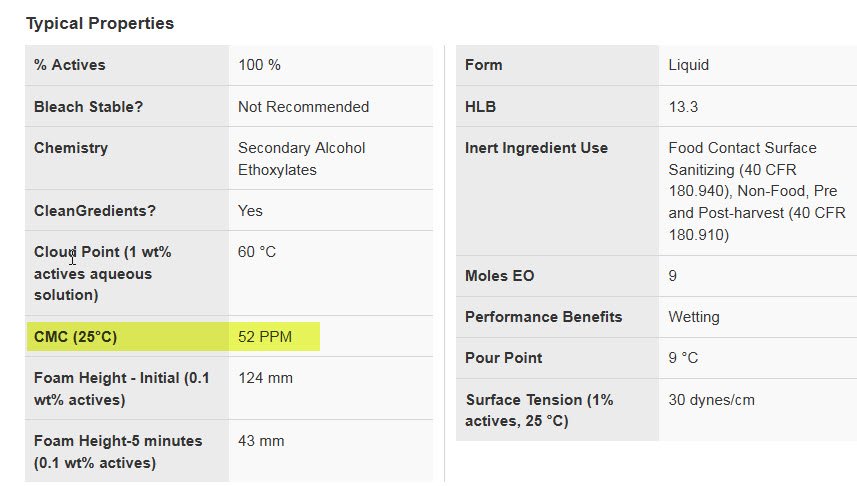
From DOW
Edit: What if any is the value of heating the rinse water?
This will be an interesting experiment.
Is that using the tank heater set at 30° C?
Yes. I set the temp to 30° C when I start the degass cycle.
Tim,I presume heating the solution has value to the extent the surfactant is more effective at a higher temp than room temperature. Here is a question for Neil (apologies if your book has the answer): At what temperature is Tergitol 15-S-9 most effective and does its effectiveness rise as the solution moves toward that temperature?
Technically, per Table VII of the book, " Cloud point is the temperature when the mixture starts to phase-separate, and two phases appear, thus becoming cloudy. The cloud point is the optimum temperature for detergency, but above the cloud point the surfactant comes out of solution and detergency drops." The cloud-point for Tergitol 15-S-9 at 1% is 60C/160F. However, at lower concentrations the cloud point will generally increase; the amount it increases is surfactant specific.
Technically as the temperature increases from 25C to 60C towards the cloud point, the CMC will decrease. The amount is specific to every surfactant, but 10% should be common. So, if the CMC is decreasing, and the concentration is >CMC then more micelles are produced at higher temps.
BUT, at/near the cloud point you may end up filtering out the surfactant, and rinsing can become a problem; so ideally to obtain the best you would operate close to the cloud-point. Also, elevating the temperature allows oily type contaminants to become more viscous and flow better which aids in removing these types of contaminants from the surface.
However, "XIV.2.a Temperature: For water, beyond about 40°C/104°F, the cavitation intensity can decrease.". Lower kHz (60-kHz) cleaning efficiency can increase by about 10% up to about 60°C/140°F. Cleaning efficiency is the combination of cavitation intensity and cleaning ability of the fluid. However, for higher frequency (132- kHz) units, cleaning efficiency peaks at 40°C/104°F and then decreases rapidly.
But records are temperature limited, so, its all a balance to get the best cavitation intensity with the best wetting/detergency with the lowest concentration otherwise you compromise the process with excess foam and then rinse tank burden. BUT, most of what you are trying to clean in your UT tank is particulate, so detergency is not as important - you want to optimize wetting and cavitation intensity.
If you need to degrease a grungy record - use a general detergent such as Alconox Liquinox (1% solution) with a vacuum RCM or manual hand scrub with a label protector. Or, you can try a wide range emulsifying cleaner - they work by soaking and dissolving target contaminants - common laundry detergents are a combination of surfactants and enzymes as is Alconox Tergazyme techbull_tergazyme.pdf (alconox.com). There are 4 basic enzymes used and how each works can be contaminant, time, concentration and surface dependent, and they have to be rinsed.
- Proteases break down protein-based soils including blood, urine, food, feces, wine and other beverages. This is the one most commonly used in cleaners.
- Amylases break down starch molecules like eggs, sugars, sauces, ice cream, gravy. This is a commonly used enzyme in cleaners.
- Lipases break down fat molecules like oils and grease. This may work for fingerprints, but mineral-based such as refined/synthetic oils/greases - not so well.
- Cellulases are used to soften fabric and restore color to fibers made up of cellulose material. They also remove particulate soil and reduce fabric graying and pilling. How well they actually remove particulate is unknown - literature is pretty thin, and likely surface dependent - may work clothes, but not hard surfaces or very small particles.
Neil
However, "XIV.2.a Temperature: For water, beyond about 40°C/104°F, the cavitation intensity can decrease.". Lower kHz (60-kHz) cleaning efficiency can increase by about 10% up to about 60°C/140°F. Cleaning efficiency is the combination of cavitation intensity and cleaning ability of the fluid. However, for higher frequency (132- kHz) units, cleaning efficiency peaks at 40°C/104°F and then decreases rapidly.
But records are temperature limited, so, its all a balance to get the best cavitation intensity with the best wetting/detergency with the lowest concentration otherwise you compromise the process with excess foam and then rinse tank burden. BUT, most of what you are trying to clean in your UT tank is particulate, so detergency is not as important - you want to optimize wetting and cavitation intensity.
Thus far I (and I believe David as well) have kept top temperatures in the 30°C - 35°C range. The idea being to assist the cleaning ability of the fluid while not damaging the vinyl.
I read that beyond 60°C vinyl records will warp. (The maximum heating plate temperature of a Furutech LP Flattener DF-2 is: 57?±3? ) I speculate that temperature lower than that (and some solvents) can soften certain areas on a vinyl record, such as groove ridge peaks - again speculation on my part - which may or may not re-harden to their original shape.
All of this as part of my wondering if increasing maximum temperature in the USC tank can yield a better cleaning process while remaining safe? For example, 40°C at 37kHz and 45°C at 80kHz.
As you say we're primarily trying to remove particulate, so detergency is less important. I enounter little grease or fingerprints on my records - at least that I know about. Then again, as you note, contaminants can mask particulate.
Optimal tank state for optimal cleaning while erring on the side of safety - yes, it is a balancing act. Imo, it is worth having some sense of state-of-art with off-the-shelf components - a model - that we can point at, given what we know. Over time we have refined the tools, the components (tanks, filters, etc.). I wonder if we can refine the process further.
Tim,Thus far I (and I believe David as well) have kept top temperatures in the 30°C - 35°C range. The idea being to assist the cleaning ability of the fluid while not damaging the vinyl.
I read that beyond 60°C vinyl records will warp. (The maximum heating plate temperature of a Furutech LP Flattener DF-2 is: 57?±3? ) I speculate that temperature lower than that (and some solvents) can soften certain areas on a vinyl record, such as groove ridge peaks - again speculation on my part - which may or may not re-harden to their original shape.
All of this as part of my wondering if increasing maximum temperature in the USC tank can yield a better cleaning process while remaining safe? For example, 40°C at 37kHz and 45°C at 80kHz.
As you say we're primarily trying to remove particulate, so detergency is less important. I enounter little grease or fingerprints on my records - at least that I know about. Then again, as you note, contaminants can mask particulate.
Optimal tank state for optimal cleaning while erring on the side of safety - yes, it is a balancing act. Imo, it is worth having some sense of state-of-art with off-the-shelf components - a model - that we can point at, given what we know. Over time we have refined the tools, the components (tanks, filters, etc.). I wonder if we can refine the process further.
Temperature can improve the cleaning efficiency - the mechanism for a record may get complicated by a thermal property called the Coefficient of Linear Expansion and in english units is referred to as in/in/F; and for PVC its about 0.000032-in per in per F; and this can also be expressed as 0.81 microns/in/F. So if the record heats from ambient (25C/77F) to 35C/95F, the record will expand by (0.81 microns/in)(18F) = 14.5 microns/in. If we were to assume the record 12" diameter, the record could expand in diameter by (14.5 microns/in)(12-in) = 174 microns = 0.007-in. You will not see this, but expanding the record 'may' loosen tightly adherent particles. FYI - metals do not expand anywhere near this amount.
However, there is another property of plastics known as the Glass Transition Temperature (Tg), and this is the temperature the polymer structure turns “viscous liquid or rubbery" - it gets soft. The Tg for rigid PVC is ~60C, plasticized PVC is ~50C, and I suspect the record PVCa is ~55C (131F). I have a Vinylflat that I made my own heater (aluminum plate and a 25W heating element) and 131F for an extended length of time (i.e.1hr) can damage a record (distortion & noise) - I do not exceed 124F (51F); but I do a very slow heat-up/cool-down.
The closer you get to the glass transition temperature with high powered ultrasonics you may enter a zone where you can do damage noting that records can have a range of composition so that the glass transition temp could be closer to 50C for some records. My recommendation for max temp would be 35C(95C) @ 37kHz (noting that this frequency has the highest risk of damage) and 40C (104F) @80kHz with the goal to operate 100% power (with pulse) @80kHz. You want a comfortable 'safety' margin between your UT tank temp and the Tg.
Good luck,
Neil
Tim,
Temperature can improve the cleaning efficiency - the mechanism for a record may get complicated by a thermal property called the Coefficient of Linear Expansion and in english units is referred to as in/in/F; and for PVC its about 0.000032-in per in per F; and this can also be expressed as 0.81 microns/in/F. So if the record heats from ambient (25C/77F) to 35C/95F, the record will expand by (0.81 microns/in)(18F) = 14.5 microns/in. If we were to assume the record 12" diameter, the record could expand in diameter by (14.5 microns/in)(12-in) = 174 microns = 0.007-in. You will not see this, but expanding the record 'may' loosen tightly adherent particles. FYI - metals do not expand anywhere near this amount.
However, there is another property of plastics known as the Glass Transition Temperature (Tg), and this is the temperature the polymer structure turns “viscous liquid or rubbery" - it gets soft. The Tg for rigid PVC is ~60C, plasticized PVC is ~50C, and I suspect the record PVCa is ~55C (131F). I have a Vinylflat that I made my own heater (aluminum plate and a 25W heating element) and 131F for an extended length of time (i.e.1hr) can damage a record (distortion & noise) - I do not exceed 124F (51F); but I do a very slow heat-up/cool-down.
The closer you get to the glass transition temperature with high powered ultrasonics you may enter a zone where you can do damage noting that records can have a range of composition so that the glass transition temp could be closer to 50C for some records. My recommendation for max temp would be 35C(95C) @ 37kHz (noting that this frequency has the highest risk of damage) and 40C (104F) @80kHz with the goal to operate 100% power (with pulse) @80kHz. You want a comfortable 'safety' margin between your UT tank temp and the Tg.
Good luck,
Neil
Excellent post with v. helpful information, Neil - thank you. I will try your recommendation later this evening.
Is this an argument for using, or at least experimenting with, our flatteners as part of the cleaning process...?You will not see this, but expanding the record 'may' loosen tightly adherent particles. FYI - metals do not expand anywhere near this amount.
I do not think so.Is this an argument for using, or at least experimenting with, our flatteners as part of the cleaning process...?
The expansion that occurs during cleaning is not constrained so the record can expand/contract freely. So, in essence the surface under the particle is moving at the same time there is high fluid agitation from the ultrasonics.
During the flattening process the record is constrained top & bottom which will also partially constrain the record side to side. The coefficient of expansion of the record is some 10X greater than metal and glass, so as the record is heated pressure/force on the record increases. The actual temperature on the record surface may be higher than what is indicated because of the pressure that may develop.
The particle is very unlikely to expand any significant amount but the record material its attached to is heating and trying to expand, but is mostly constrained and you will be very close to the glass transition temperature. If anything, as the record heats up constrained and softens, this may more deeply adhere particles into the grooves. If anything its a good reason to make sure the record is as clean as possible before flattening.
Neil
Indeed. I always wash records prior to flattening for this very reason. I have no evidence that this occurs, but simple intuition suggesting it might.If anything, as the record heats up constrained and softens, this may more deeply adhere particles into the grooves. If anything its a good reason to make sure the record is as clean as possible before flattening.
Hi everybody,
I setup the rinse US and did a test batch today. Compared to my previous vacuum rinse the speedup of the whole process is fantastic. I did not do any listening tests but given the speedup I won't comeback to the vacuum rinse unless something bad is happening. The first obvious observation of the process is that the DI does not wet the vinyl as efficiently as in the wash tank. There are also macroscopic bubbles appearing in the tank from time to time. Neil, does it make sense to add say 3% of ethanol to the rinse tank to improve wetting (I was using 3% ethanol for the vacuum rinse with good results)?
The process at the moment:
1. Vacuum pre-clean: 5% IPA + 0.05% Tergitol S7
2. Main US wash: 240W/10l tank, 5 vinyls, 1rpm, 2.5%IPA + 0.02% S7, 10mins, 35C-38C, 100% power
2.5 Letting drip and dry
3. US rinse: 150W/6l tank, 3 vinyls, 1rpm, DIW, 7mins, 35C-38C, 100% power
The filtration is still not 100% ready - waiting for the 0.2um filter from China, they are unobtanium here in EU and UK ships for the price of gold. At the moment the rinse tank is unfiltered, the main tank uses the current 0.5um nominal with weak pump.
Cheers,
I setup the rinse US and did a test batch today. Compared to my previous vacuum rinse the speedup of the whole process is fantastic. I did not do any listening tests but given the speedup I won't comeback to the vacuum rinse unless something bad is happening. The first obvious observation of the process is that the DI does not wet the vinyl as efficiently as in the wash tank. There are also macroscopic bubbles appearing in the tank from time to time. Neil, does it make sense to add say 3% of ethanol to the rinse tank to improve wetting (I was using 3% ethanol for the vacuum rinse with good results)?
The process at the moment:
1. Vacuum pre-clean: 5% IPA + 0.05% Tergitol S7
2. Main US wash: 240W/10l tank, 5 vinyls, 1rpm, 2.5%IPA + 0.02% S7, 10mins, 35C-38C, 100% power
2.5 Letting drip and dry
3. US rinse: 150W/6l tank, 3 vinyls, 1rpm, DIW, 7mins, 35C-38C, 100% power
The filtration is still not 100% ready - waiting for the 0.2um filter from China, they are unobtanium here in EU and UK ships for the price of gold. At the moment the rinse tank is unfiltered, the main tank uses the current 0.5um nominal with weak pump.
Cheers,
Last edited:
There are also macroscopic bubbles appearing in the tank from time to time. Neil, does it make sense to add say 3% of ethanol to the rinse tank to improve wetting (I was using 3% ethanol for the vacuum rinse with good results)?
Jarek,
For you step 2.5, I suggest you do not allow it to dry. Shake to remove bulk water and move to rinse. You will get some cleaner carryover - even if you let it dry. 0.02% Tergitol = 200 ppm = 200 mg/L = 0.2 mg/L. If each side of the record holds 1.5-mL of liquid, 3 mL is carried over to the rinse tank = 0.6 mg of Tergitol; diluted in the rinse tank to 6 L = 0.1 mg/L = 0.1 ppm. So every record increases the Tergitol in the rinse tank by ~0.1 ppm. By the time you clean 100 records - the rinse tank will reach about 10 ppm Tergitol in the rinse tank, and you may notice the improved wetting. The residue thickness from water with 10 ppm Tergitol drying on the record is inconsequential. So, refresh the rinse tank after ~100 records, or if you see the records wetting a lot, or if TDS reaches 5-10 ppm noting that TDS measures only ionic impurities so it will not measure nonionic surfactants such as Tergitol.
The 0.02% (200 ppm) Tergitol 15-S-7 in the wash tank reduces the surface tension of the water from 72 dynes/cm to 28 dynes/cm - this will wet the record instantly. If you add 3% ethanol (or isopropyl) the surface tension of the water is going to decrease about 15%, so it will drop from 72 dynes/cm to about 60 dynes/cm; and for isopropyl Figure 33 in the book shows this. This will only marginally effect the wetting. The biggest change is that the boiling-temp (vapor pressure) drops by 12degC and you can see what happens with Isopropyl in Figure 32. This drop in vapor pressure is a double edged sword because it speeds up the drying but it also reduce the cavitation intensity.
In the rinse tank we are generally not worried with surface tension, we want the maximum cavitation energy because that it going to both wet the record (when submerged) as well as setup the agitation to remove any cleaner residue and last remnants of particles.
Also, and I am repeating myself, but where the book says that isopropyl (and ethanol) forms an azeotrope with water is only true for >50% concentration. At less, the alcohol will separately evaporate from the water, so with a large open heated UT tank, the alcohol concentration will be decreasing (over some time) unless you are monitoring with an ethanol (or isopropyl) hydrometer and adjusting. Because of this detail of water/alcohol, I am no longer recommending alcohol for UT tanks that are being operated to extend bath life unless:
-For the wash tank, if you are going to use IPA, you should monitor the IPA concentration and supplement to keep the concentration 2.5-5%.
-For the rinse tank, you can try ethanol and see if that improves the end product sufficient to warrant the burden of now having to monitor the alcohol concentration or refresh the rinse tank frequently enough to mitigate.
Devil is in the Details.
Neil
Neil, your knowledge is infinite! Thank you!
Great calculation of the contamination. I think it is even less than 3ml that each vinyl carries after shaking the cleaner off. Removing the drying stage will speed up the process even more.
I also stop bothering about the wetting and adding ethanol to the rinse tank, the cavitation action is more important.
Cheers,
Great calculation of the contamination. I think it is even less than 3ml that each vinyl carries after shaking the cleaner off. Removing the drying stage will speed up the process even more.
I also stop bothering about the wetting and adding ethanol to the rinse tank, the cavitation action is more important.
Cheers,
he biggest change is that the boiling-temp (vapor pressure) drops by 12degC and you can see what happens with Isopropyl in Figure 32. This drop in vapor pressure is a double edged sword because it speeds up the drying but it also reduce the cavitation intensity.
To correct myself, as the boiling point drops, the vapor-pressure increases.
You will get some cleaner carryover - even if you let it dry. 0.02% Tergitol = 200 ppm = 200 mg/L = 0.2 mg/L.
200 mg/L = 0.2 mg/mL
-For the rinse tank, you can try ethanol and see if that improves the end product sufficient to warrant the burden of now having to monitor the alcohol concentration or refresh the rinse tank frequently enough to mitigate.
Edit: I always wondered if IPA was the 'secret ingredient' in Lloyd Walker's 'Ultra Pure Final Rinse', part of his Prelude system, pre-ultrasonic. Since we are rack drying, I might try it. The trade-off, as you say, between reduced drying time and cavitation intensity. Do you have a suggestion for a good balance?
Last edited:
Tim,Edit: I always wondered if IPA was the 'secret ingredient' in Lloyd Walker's 'Ultra Pure Final Rinse', part of his Prelude system, pre-ultrasonic. Since we are rack drying, I might try it. The trade-off, as you say, between reduced drying time and cavitation intensity. Do you have a suggestion for a good balance?
Because of liability concerns, I will not recommend a concentration above 2.5%. However if you review Figures 31 and 32 the best concentration to get the maximum decrease in boil point and still have a very safe (20F) flammability margin is obvious. But, it will decrease the cavitation intensity, but that 'may' be offset by the lower surface tension; toxicity concerns notwithstanding.
IPA odor threshold is ~22 ppm, so if it was in Lloyd Walker's 'Ultra Pure Final Rinse', you should smell it. However, Ethanol has an odor threshold of ~80 ppm. The allowable exposure limit for IPA by OSHA is 400 ppm, but the American Conference of Governmental Industrial Hygienists (ACGIH) sets exposure limits for IPA at 200 ppm. The allowable exposure limit for ethanol is 1000 ppm.
As you increase your throughput, if you use a solvent such as IPA/Ethanol, knowing that it is going to evaporate from the water and knowing that the vapor concentration in the air is going to increase, you should review the area (volume) you are working and the ventilation rate (turn-over) to make sure you are doing no harm to yourself or your family. The toxicity risk from ethanol is pretty low but IPA is different, and know that for medical (hospitals) they have engineered ventilation systems to generally provide about a 15-20 min turn-over (air is exchanged with fresh air every 15-20 min). Residential homes do not have this kind of ventilation.
As you increase your throughput, the cleaning process steps ever closer to a full industrial process with all the benefits but also all the risks & concerns. In an industrial throughput environment the records would be dried under a tox-hood that would ventilate to outside, or an air filter with activated carbon/charcoal which can absorb alcohols (and other volatile compounds) - Microsoft Word - TIB Activated Carbon Adsorption List.doc (ipsystemsusa.com) would be used. PS/there are air filters with activated carbon that can be used for your HVAC - be careful with these, they have very high pressure drop and can significantly reduce the life of the HVAC system.
Devils in the details,
Neil
PS/Thanks for typo catch; its why I always show my math.
In an industrial throughput environment the records would be dried under a tox-hood that would ventilate to outside, or an air filter with activated carbon/charcoal which can absorb alcohols (and other volatile compounds) - Microsoft Word - TIB Activated Carbon Adsorption List.doc (ipsystemsusa.com) would be used.
Interesting you mention air filtration. For a few weeks now I've place a small air filter next to my air drying station. Filtration includes H13 'true' HEPA and activated carbon. Unit and filters are relatively inexpensive.
I run it for ~4-6 hours prior to a cleaning session. It is spec'd for 219 sq ft whereas my room is 312 sq ft. I"m simply trying to keep stray particulate down as records dry, not that I have an issue with that, but the thing was not in use so figured to try it out. I understand your reference is pertinent to the wash/rinse tanks where IPA might be in use.
Similar threads
- Replies
- 3
- Views
- 318
- Replies
- 7
- Views
- 1K
- Replies
- 13
- Views
- 535
- Replies
- 21
- Views
- 2K
| Steve Williams Site Founder | Site Owner | Administrator | Ron Resnick Site Owner | Administrator | Julian (The Fixer) Website Build | Marketing Managersing |





